Three States of Matter
This lesson is about states of matter for kids. Learn facts about states of matter.
Matter is all around us. All materials that we know can be grouped as solids, liquids and gases.
Examples of matter around us
These groups are called the three states of matter.
Examples of three states of matter
- Wood ➡ Solid
- Water ➡ Liquid
- Air ➡ mixture of gases
Most of the substances on Earth are solids at everyday temperatures. Therefore, only a few substances are liquids or gases compared to the amount of solids.
|
Properties of solids liquids and gases |
|||
|
Properties of Solids |
Properties of Liquids |
Properties of Gases |
|
| Definite shape (A solid can be cut or shaped only when an external force acts upon it.
E.g.
The properties of many solids make them useful materials for manufacturing items. |
Indefinite shape (A liquid takes the shape of the container which it is kept)
Put the same volume of water into each container. You can see how water takes the shape of the different containers it’s put into. |
Indefinite shape (A gas spreads all over the place to fill up the space which it is kept) | |
| Definite volume | Definite volume | Indefinite volume | |
| Highest density | Density is lower than solid | Lowest density | |
| Rigid and cannot flow | Can flow. Flows to the lowest point that it can, so that it fills the bottom part of a container.
Can flow |
||
| Greatest force of attraction among particles | Less force of attraction among particles compared to solids | Least force of attraction among particles | |
| Particles are tightly packed and cannot move freely. | Particles are loosely packed compared to solids and can move freely by sliding over one another. | Particles are very loosely packed. The particles are in a continuous random motion because the gaps among particles are the greatest. | |
| Cannot be easily compressed (squashed), expanded or changed | Cannot be compressed (squashed) | Can be easily compressed (squashed) | |
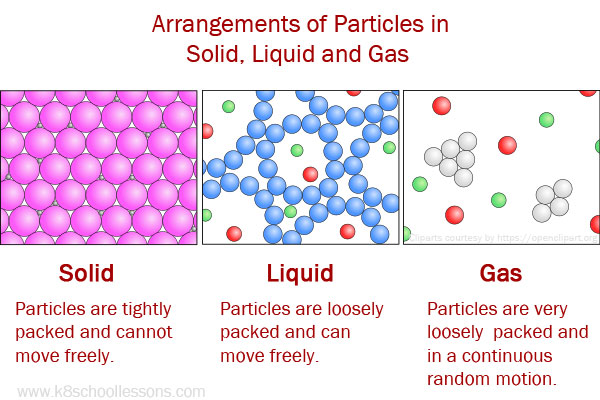
Due to the property fluidity, gases and liquids are called fluids, because they both can flow.
Plasma
Plasma is the fourth state of matter. It rarely exists naturally on Earth. We’ll later discuss about the state plasma.
Solid to liquid to gas
We identify substances as solids, liquids and gases in everyday conditions. This normal condition is often at the room temperature, which is the temperature inside a building and under normal atmospheric pressure. Various substances can exist in all three states of matter. The best example is water.
Three states of water
- Water can be a solid ➡ Ice
- Water can be a liquid ➡ Water
- Water can be a gas ➡ Water vapour or steam
Learn how materials change their state with this lesson, states of matter for kids.
Changing States
Materials can be changed from one state to another by heating or cooling (freezing). When a substance changes from one state to another a change of state happens.
The most common substance, water as an example;

Freezing Water and Melting ice

Heating Water and Cooling Water Vapour
Hope you’ve enjoyed the lesson states of matter for kids. Also, try the activities ‘Solid, Liquid and Gas‘ and ‘States of Matter Quiz‘